Drug Test Results
Drug testing is a multi-step process: data from each step, such as specimen collection, lab results, and follow-up on positives, are all entered separately. Accessing the Drug Test screen from Orders and/or a form is suitable for initial data entry (through specimen collection).
Federally regulated drug tests require a printed requisition form to accompany the specimen. Electronic requisitions are acceptable for non-DOT drug tests. Subsequent results data entry is either done via direct import or from the order/form.
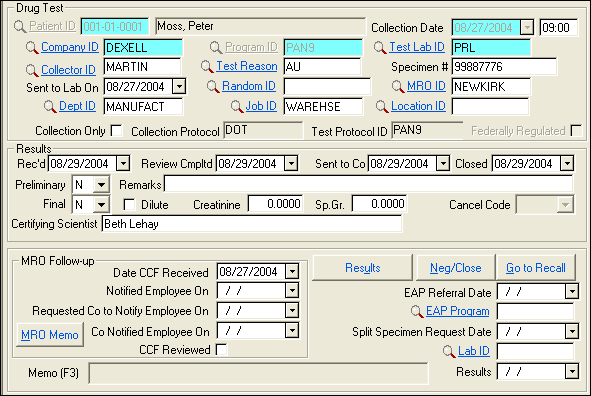
| Label | Description |
|---|---|
| Collection Date | Date of specimen collection. |
| (Unlabeled adjacent field) | Time of specimen collection in 24-hour format. |
| Company ID | Patient's employer at time of test,
entered by
SYSTOC automatically. Overwrite
with appropriate Company ID if this test is for a different company (e.g.,
pre-employment).
Note: If you are using LabLink to import
drug test results, do not use Company IDs that contain hyphens; they will not
import correctly.
|
| Program ID | ID of the company program that specifies the way a patient is tested. There must be a company program before drug test data can be entered. Select from the look-up. |
| Test Lab ID | ID code for lab performing analysis; may fill automatically from Program ID. |
| Collector ID | ID code for person monitoring collection. |
| Test Reason | ID code to explain reason and/or situation for testing. Use F5 to select from the lookup. If this is a random test, be sure to complete the Random ID field also. |
| Specimen # | Identification numbers/letters on specimen. |
| Sent to Lab On | Date specimen sent to lab for analysis, press F8 for today. |
| Random ID | If this test was performed because of the randomization of a pool/consortium, specify the ID of the randomization that selected the employee. Use the Lookup to select the number. Normally, the Random ID at the top of the list will be the correct one, assuming the sample was taken within a reasonable amount of time following selection. If in doubt, scroll the lookup list to the right to check the dates. Failure to enter the Random ID will cause problems when reports are run for that randomization: it will look like the person was never tested. |
| MRO ID | Med Staff ID code for the Medical Review Officer; may fill automatically from Program ID . The lookup (F5) list shows only staff who are MROs. |
| Dept ID | ID for patient's department at work; fills automatically. Correct if needed. |
| Job ID | ID for patient's job; entered by SYSTOC automatically. Correct if needed. |
| Location ID | Location ID for this test. |
| Collection Only | Check if your clinic is simply collecting the specimen at the request of another facility. |
| Collection Protocol | ID for collection protocol being followed. Entered by SYSTOC automatically, based on the selected Program ID. |
| Test Protocol ID | Test Protocol ID associated with the Program ID selected; fills automatically. |
| Federal Regulated | Will be checked if the Test Protocol is checked to indicate it is a federally-regulated protocol. This is to alert you, and to allow reports to screen for federally-regulated test results. |
| Order ID | Does not appear on the screen; it is located on the far right of the search grid. |
| Results | |
| Rec'd | Date test results are received. Enter this date and save the record before using either the Results or Neg/Close buttons. |
| Review Cmpltd | Date medical staff finishes review of results. When this field is blank, the results appear on the MRO Analysis Form report, which is used to determine which results need to be reviewed. Closing the case automatically completes this field with today's date. |
| Sent to Co | Date clinic gives final results to company. Closing the case automatically completes this field with today's date. |
| Closed | Date of case closure. Closing means results are finalized and ready to be reported. This date fills automatically if you reply Yes to the "Close Case" question that displays when final results are entered. |
| Preliminary & Final |
Only X, S, A, or a blank may be entered in the Preliminary field. Other entries are controlled by detailed results entered via the Results or Neg/Close buttons. See also Enter Results Manually for details about adding other results. Note: If your clinic imports lab test results, the import adds the
preliminary results and corresponding numeric values. See also
Results via the HL7 Drug Test Interface
|
| Remarks | If the preliminary result is A, S, or X, you are required to explain in this field that prints on result reports. |
| Dilute | Indicates if the specimen is reported as dilute by the lab. |
| Creatinine | Indicates if the specimen results contain a creatinine level. |
| Sp. Gr | Indicates if the specimen results contain a specific gravity level. |
| Cancel Code | If the result is canceled, select a code from the drop-down list (codes defined in system message field at top of SYSTOC screen). |
| Certifying Scientists | Reviews and attests to the accuracy and validity of the drug test results reported by the laboratory. The scientist shall have a thorough understanding of chain of custody procedures and drug testing regulations. |
| MRO Follow-up | |
| Date CCF Received | Date MRO received copy two of the Custody and Control Form (CCF). Autopopulates with the collection date if the indicated MRO has an S in the Staff field of the Medical Staff file. |
| Notified Employee On | Date contact is made with employee regarding positive results. |
| Request Co Notify | Date clinic asks company to contact employee. This is necessary if the clinic is unable to contact the employee directly for an explanation of an initial positive result. |
| Co Notified Employee | Date company actually told employee to contact clinic for MRO review. |
| CCF Reviewed | Check this box if the MRO has reviewed this drug test. Results marked in this way count toward the requirement to review 5% of the results. |
| EAP Referral Date | Date employee is referred to Employee Assistance Plan (EAP). |
| EAP Program | ID code of Employee Assistance Plan, if any. |
| Split Spec. Request Date | Date employee requests split specimen testing. |
| Lab ID | ID code for lab that tests the split specimen. |
| Results | Date results of split specimen testing are received. These fields must be completed before adding the results of the split test. |
| MRO Memo | This button launches a memo for the MRO to use for notes on the case. |
| Memo (F3) | Press F3 to access this memo. Refresh your screen after making or modifying an entry to see the first line of the memo in this field. A Memo Changed column in the List view indicates when the last modification was made. |
| Buttons | |
| Results | Use for entering positive results, see Positive Results. |
| Neg/Close | Use this button to enter negative
results for the entire panel (each test performed) and close the case. This
action enters today's date in the Closed, Review Cmpltd, and Sent to Co fields,
and places an
N in
both the Preliminary and Final fields. You can change the (default) date
entries, but cannot change the results without reopening the case.
Note: When
Negative drug test results (for
all substances) are imported and you have the Program Preferences
Auto close drug
tests on HL7 imported Negative results option selected, then the
drug test will be marked closed automatically.
|
| Go to Recall | This button is provided as a convenience, to create a Recall record, if needed, for reminding the patient to return at a later date. |