Company Drug Programs
The company drug program defines how drug tests are conducted.
Use the form Company Profile/Drug Test Program in the SYSTOC Copy-Ready Forms packet from the Customer Resource Center: http://ul.custhelp.com (login required) to collect information on the requirements of each company.
To simplify data entry, create a generic company
drug program that contains your most frequently used Test Lab, MRO, Results Due
Back, etc. Copy and customize this generic program for each client.
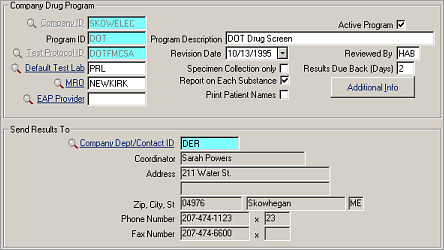

| Label | Description |
|---|---|
| Company ID | ID of the company using this program. |
| Active Program | Indicates the program's status. When the checkbox is selected, the status is Active. On an add record, this is selected by default. This is especially useful when excluding inactive programs in search lists and reports. The search defaults to show active programs. |
| Program ID | A unique ID for the program. A company can have multiple drug programs. |
| Program Description | Name of the program. |
| Test Protocol ID | The code for the appropriate panel of tests and collection method, see Test Protocols. |
| Revision Date | Most recent change to drug program. |
| Reviewed By | Initials of person approving the program. |
| Default Test Lab | ID of the test lab (from ). Leave blank if more than one lab will be used. Must be certified if the Test Protocol requires it. If using the HL7 Drug Test Interface to receive drug test results, specify a Lab and its account information. See Laboratory Account Information. |
| Specimen Collection Only | Select the checkbox if the clinic will collect specimens only. |
| Results Due Back (Days) | Number of days to receive results from the lab, used in the Drug Screen Tracking report. |
| Additional Info | Opens Company Drug Program - Additional Information. See Company Drug Program - Additional Information |
| MRO | ID of the Medical Review Officer (MRO). Leave blank if more than one MRO reviews results. If you don't see the name you expect in the lookup, be sure that the MRO field in the Medical Staff record is checked. |
| Report on Each Substance | Check the box if this is DOT-mandated testing, as individual substance reporting is required. |
| EAP Provider | Employee Assistance Plan provider, if any. |
| Print Patient Names | Controls whether the patient name appears on the Drug Screen Results Letter for the company; does not apply to the summary report for the DOT, which never contains names. Names are required for DOT testing. This field also controls whether patient names appear on the iSYSTOC Drug List and Drug Detail screens. |
| Send Results To | For Company Dept/Contact ID field in this section, select the DER (Designated Employer Representative) contact that should exist for each company, see Departments/Contacts. |