Test Protocols
A test protocol describes a complete testing procedure for a drug substance or group of substances.
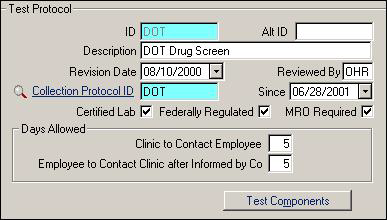
| Label | Description |
|---|---|
|
ID |
Use an easy-to-remember, descriptive ID code. |
|
Alt ID |
The Alternate ID is for electronic transmission. Do not use it for any other purpose. When you sign up for electronic transmission, the appropriate Alternate ID will be provided. |
|
Description |
Full description of test protocol. |
|
Revision Date |
Optional, date of most recent change. |
|
Reviewed By |
Optional, initials of person who most recently checked/revised protocol. |
|
Collection Protocol ID |
ID of Collection Protocol to use. |
|
Since |
Optional, date this Collection Protocol was selected. |
|
Certified Lab |
Check if a certified lab is required. This limits the list of labs when selecting labs from the lookup on the Drug Test Results screen. |
|
Federally Regulated |
Check this box if the protocol is for federally-regulated drug testing. Certain reports look for this factor. |
|
MRO Required |
Is a Medical Review Officer required? |
| Days Allowed | |
|
Clinic to contact Employee |
How many days shall the clinic attempt to contact an employee about positive results before asking for company help? This field and the one below are used on the Drug Screen Tracking Report. |
|
Employee to Contact Clinic |
How many days are allowed for the employee to contact the clinic after being told to do so by the company? |